Autophagy
The process of autophagy (“self-eating” in Greek) involves the degradation of bulky material (“cargo)” in double-membraned cellular compartments, called autophagosomes, that eventually fuse with lysosomes to destroy the cargo.
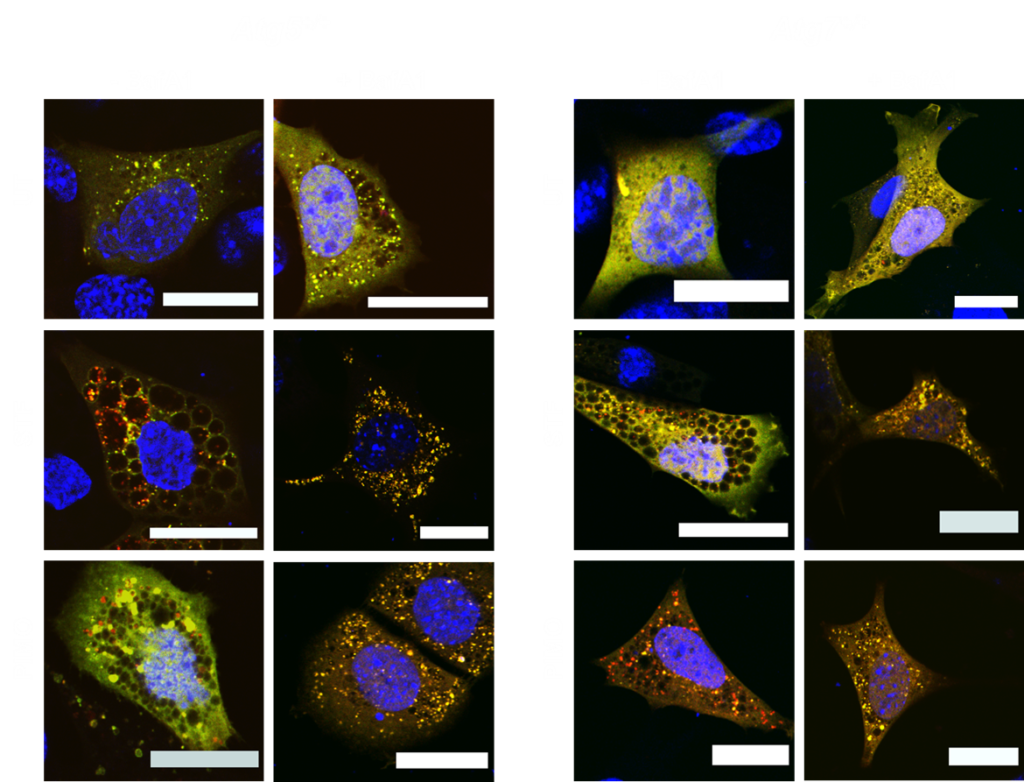
Autophagy targets damaged cellular organelles, like mitochondria (mitophagy) (see our work on ubiquitin chain-specific sensors and mitophagy in fixed and living cells: van Wijk et al 2012 and van Wijk et al 2013), endosplasmatic reticulum (ER; ER-phagy) (see Zielke et al, accepted) or ribosomes (ribophagy), aggregated cellular proteins (aggrephagy) or intracellular pathogenic bacteria, virusses and parasites (xenophagy) (see our ongoing work on ubiquitin chain-specific sensors, linear ubiquitin, OTULIN and intracellular Salmonella with conventional immunofluoresence and super-resolution imaging: van Wijk et al 2012, van Wijk et al 2013, van Wijk et al 2017, van Wijk et al 2019).
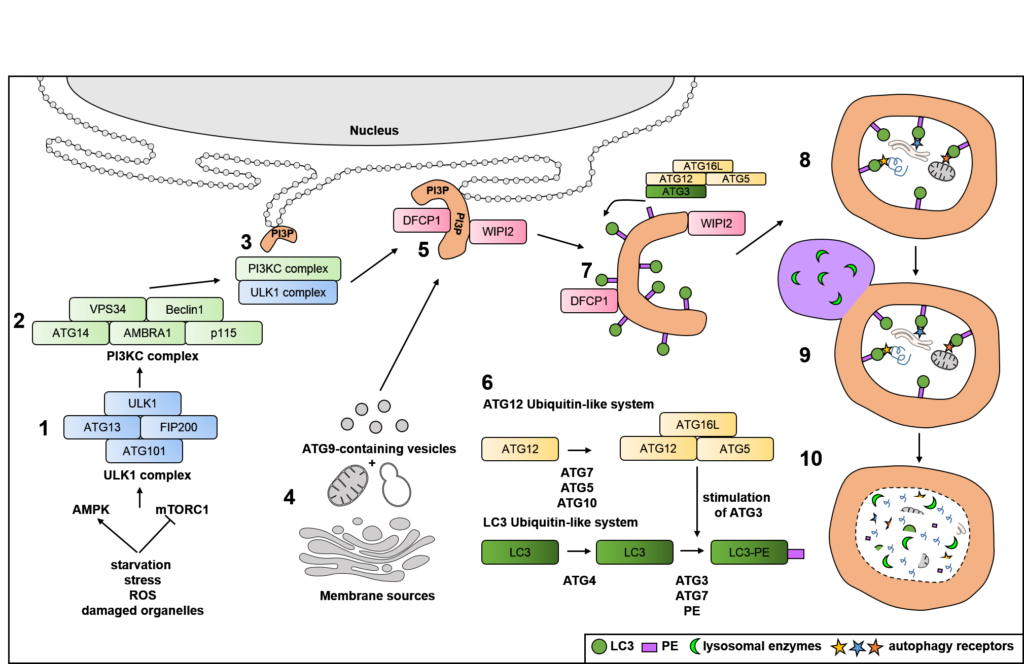
By doing so, autophagy contributes to protect cells against danger signals and recycles amino acids and lipids that are released upon lysosomal hydrolysis. Selectivity during autophagy is achieved by specialized autophagy receptors, like p62/SQSTM1, optineurin (OPTN) and FAM134B, that facilitate the recruitment of autophagic membranes to cargo and autophagy adaptors that attract the cellular machinery to autophagosomes.
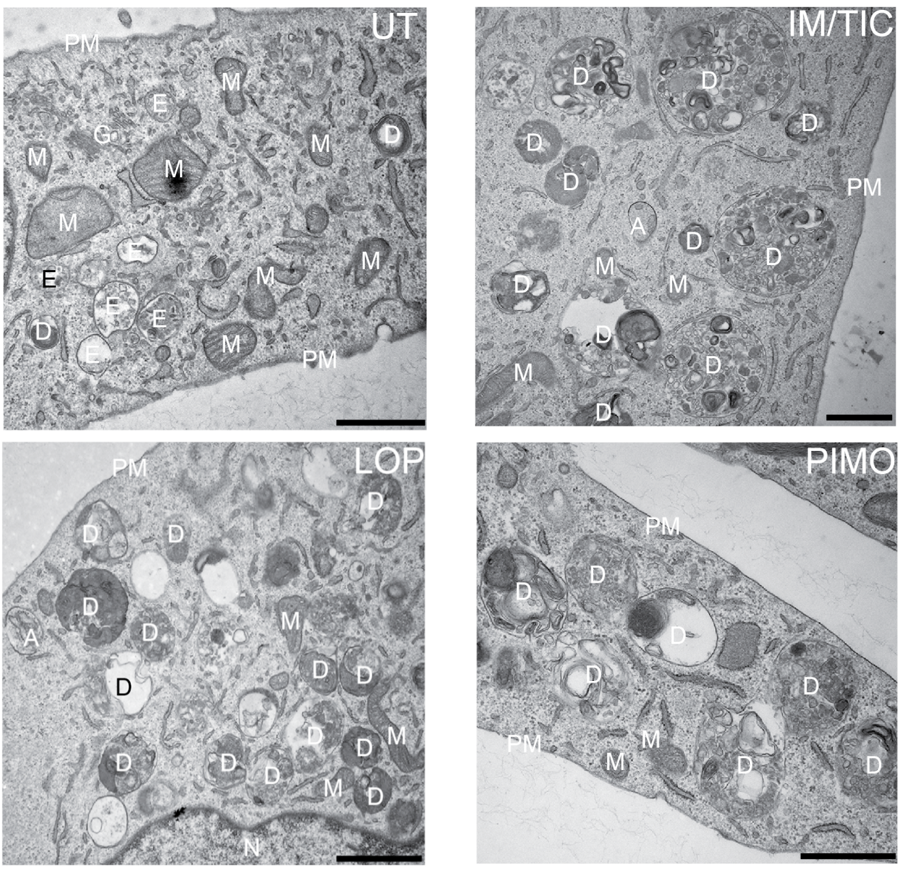
In these scenarios, autophagy serves cytoprotective roles. However, under certain conditions, extended, prolonged periodes of autophagy or hyper-activation of autophagy can induce cell death (autophagic cell death, ACD). We have studied this by screening a chemical library of autophagy-inducing substances and identified several compounds that selectively induce autophagy and caspase-independent autophagy-dependent cell death in human glioblastoma (Zielke et al 2018) and mouse embryonic fibroblasts (Kinzler et al 2020).
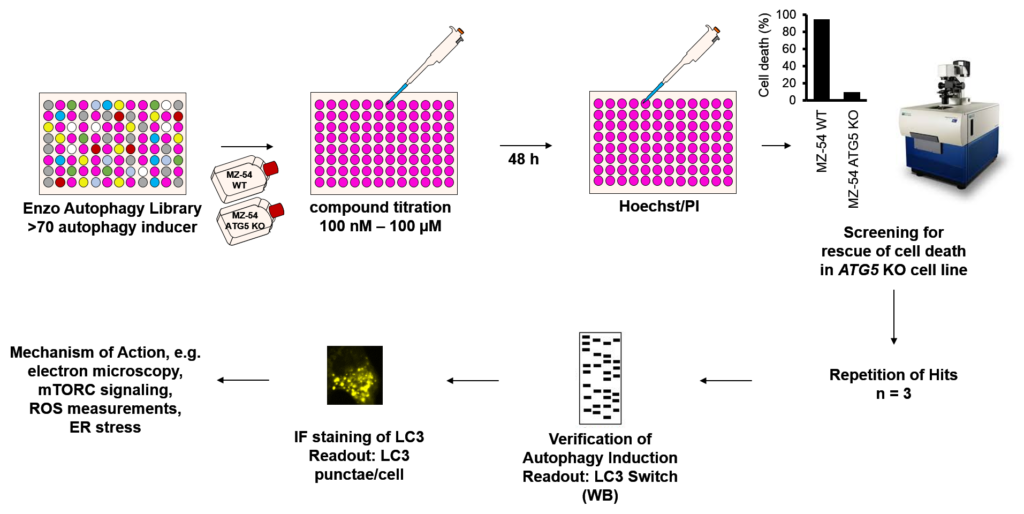
Our current work is focussing on unraveling the mechanisms of drug-induced autophagy and ACD in healthy and in cancer cells using immunofluoresence, CRISPR/Cas9 functional genomic screenings, autophagy reporters, electron microscopy and classical biochemistry.